Different elements change the color of the glass and it can give it fluorescence.
Uranium glass is a light green colored glass and glows bright green under UV light.
Uranium glass might have different colors and is not limited to being transparent, from white opaque, blue, green, orange, turquoise transparent.
Soda-lime glass appears colorless but impurities of iron oxides produce a green tint in thick pieces.
Additives to color glass:
- iron oxide makes bluish-green glass frequently used in beer bottles. Together with chromium adds a richer green color used for wine bottles.
- Sulfur with carbon and iron salts forms amber glass with yellowish to almost black color glass; with calcium it yields a deep yellow color
- Manganese in small amounts will remove the green tind given by iron or in high concentrations gives the glass an amethyst color. purple color.
- Manganese dioxide is used to remove the green color from glass or give a dark purple color to the glass/ violet.
- Small concentrations of cobalt (0,025-0,1%) gives blue glass color.
- 2 to 3% of copper oxide gives turquoise color.
- Nickel produces blue or violet and even black glass. Lead with nickel gives purplish color.
- Nickel with small amounts of cobalt was used to decolorize lead glass.
- Chromium is used to give a dark green or even black in higher concentrations.
- Chrome with tin oxides gives emerald green glass.
- Cadmium with sulfur forms cadmium sulfide and results in deep yellow color glass but cadmium is toxic. Together with selenium and sulfur has bright red and orange.
- Uranium of 0,1 to 2% was used to give a green-yellow color or other and give a yellow or green fluorescence.
- Didymium gives green color and is used in UV filters or gives lilac red color.
- Selenium like manganese, can be used in small concentrations to decolorize glass or in high concentrations to give a reddish color. When used with cadmium sulfide has a brilliant red color known as selenium ruby.
- Pure metallic copper produces a very dark red, opaque glass used as a subtitute for gold in the production of ruby-colored glass.
- Metallic gold in small concentrations of 0,001% gives a rich ruby colored glass (ruby gold)
- Silver produces colors from orange-red to yellow and also has photochromism or tenebrescence.
- Tin oxide with antimony and arsenic oxides can produce an opaque white glass like milk, was used to produce an imitation porcelain.
Lenses are commonly made of glass, but other materials like quartz glass, fluorite, plastics (acrylic plexiglass), germanium and even meteoritic glass is also used.
Quartz contains only silicon and oxygen but comercial quartz glass often contains impurities like aluminium and titanium. Fuzed quartz is prone to phosphorescence and solarisation (purple discoloraiton) under intense UV illumination. Fused quartz/fused silica properties: density 2,204; hardness 5,3-6,5; melting point 1665 C.
Fluorite glass have low dispersion and less chromatic aberration making them valuable in microscopes and telescopes.
Germania (germanium oxide GeO2) has high index of refraction and low optical dispersion making them usefull for wide-angle camera lenses in microscopy and the core part of optical fibers.
Opalescent glass appears blue and shines orange light through, effect named Tyndall effect.
Under fluorescent light tube:
Under LED source:
Under longwave UV light:
Under shortwave UV light:


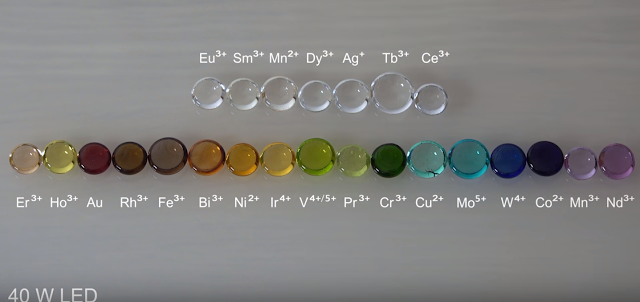


Comments
Post a Comment